Long-Term Efficacy and Safety of Selective PPARδ Agonist Seladelpar in Primary Biliary Cholangitis: ASSURE Interim Study Results
June 2025
Introduction
The objective of these analyses was to evaluate interim data from the ongoing, open-label, long-termefficacy and safety ASSURE study of seladelpar, a selective peroxisome proliferator-activated receptor δ agonist, in primary biliary cholangitis.
Methods
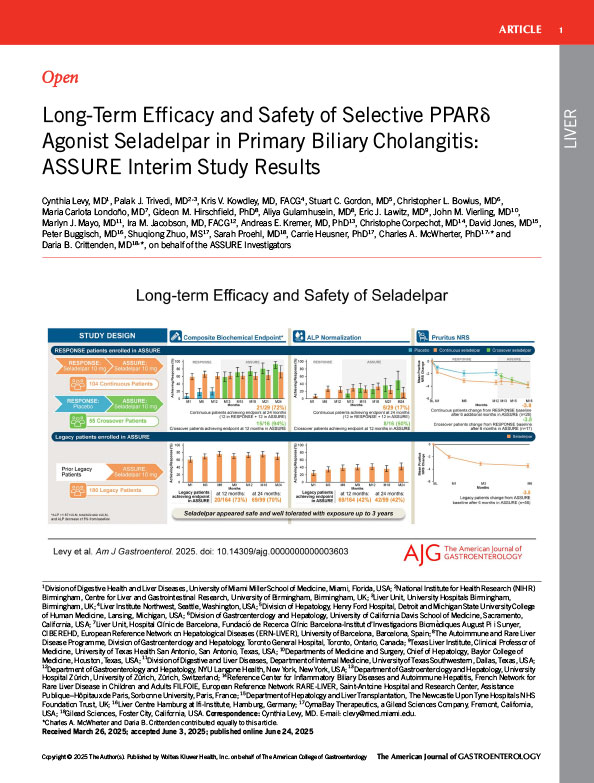
Patients rolling over from the phase 3, randomized, placebo-controlled, 12-month RESPONSE study or with previous participation in earlier legacy seladelpar studies were enrolled. Interim evaluations included composite biochemical response (alkaline phosphatase <1.67×upper limit of normal, total bilirubin £ upper limit of normal, and alkaline phosphatase decrease ‡15%), pruritus numerical rating scale (NRS) change among patients with a baseline score ‡4, and safety.
Results
At interim cutoff, 337 patients were enrolled and received ‡1 seladelpar 10 mg dose: 54 placebo-treated and 104 seladelpar-treated from RESPONSE and 179 from legacy studies. The composite response rate at RESPONSE completion was 62% (79/128) with seladelpar and 20% (13/65) with placebo. After 12 months in ASSURE, among patients who rolled over from RESPONSE, response rates were 72% (21/29) in patients continuing seladelpar and 94% (15/16) in crossover seladelpar patients. In legacy trial patients, response rates were 73% (120/164) and 70% (69/99) after 12 and 24 months of treatment in ASSURE, respectively. The NRS decrease at RESPONSE completion in seladelpartreated patients with baseline NRS ‡4 (−3.4) was maintained after 6 additional months of treatment (−3.8); changes were similar in crossover seladelpar (−3.8) and legacy patients (−3.5) after 6 months of treatment in ASSURE. No seladelpar-related serious adverse events were reported.
Discussion
Seladelpar demonstrated durable improvements in cholestatic biomarkers and pruritus in patients with primary biliary cholangitis with up to 2 years of treatment and remained overall safe with long-term use. Clinicaltrials.gov: NCT03301506.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology
Note: Obeticholic acid, marketed under the brand name Ocaliva® for the treatment of primary biliary cholangitis (PBC), was voluntarily withdrawn from the US market by Intercept Pharmaceuticals following a request from the US Food and Drug Administration (FDA) on 11/14/2025